Background: On-target off-tumor toxicities of anti-CD19 CAR T-cell therapy include B-cell aplasia and hypogammaglobulinemia. Long term B-cell aplasia is hypothesized to represent functional CAR T-cell engraftment (Melenhorst, Nature 2022), while peripheral blood B-cell recovery represents the loss of functional CAR-T cells against target over time. Patients with large B-cell lymphoma (LBCL) who are clinical responders have a 50-70% chance of B-cell recovery in the first year, with the remainder experiencing longer term B-cell aplasia (Logue, Haematologica 2021). Here, we sought to identify factors associated with long term B-cell aplasia using a cohort of responding patients who received CAR T-cell therapy for LBCL.
Methods:This retrospective cohort study included 57 patients with LBCL treated with CD19-targeted CAR T-cell therapy between June 2016 and August 2020 at Moffitt Cancer Center who exhibited complete or partial response at six months after CAR T-cell infusion. Patients were treated with with axicabtagene ciloleucel (axi-cel; n=50) or tisagenlecleucel (tisa-cel; n=7), either as standard-of-care therapy (n=47) or as part of a previously published clinical trial (n=10; NCT02348216, NCT03391466, NCT03153462). Peripheral blood B-cells were measured by flow cytometry using CD19 expression as part of routine clinical care at baseline and periodically after infusion. Prolonged B-cell aplasia was defined as a CD19+ B cell count comprising less than 1% of peripheral blood mononuclear cells during a minimum of two separate time points beyond 6 months of follow-up. Patients were defined as having B cell recovery if >1% B-cells were observed in peripheral blood at any time after CAR T-cell infusion.
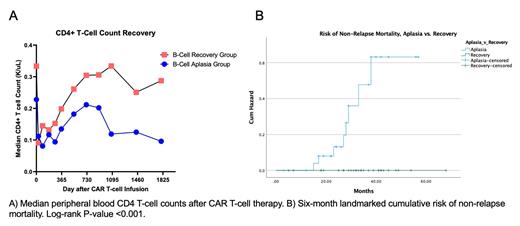
Results: At a median follow up of 33 months (95% CI: 28.7, 37.3 months), 29 (51%) patients had persistent B-cell aplasia and 28 (49%) had recovery of peripheral B-cell counts. Median B-cell count at 12 months in the recovery group was 84/µL (IQR 2.5-197) compared to 0/µL (IQR 0-1) in the aplasia group. Patients with long-term B-cell aplasia had lower CD4 T-cell counts prior to CAR T-cell infusion [median 200/µL (IQR 136-278) vs. 330/µL (IQR 224-451); P= 0.008] and over time compared to patients with B-cell recovery (Fig. A). Patients with long-term B cell aplasia also had lower pre-infusion absolute neutrophil counts (2.3K/µL (IQR 1.4-4.15) vs. 3.5K/µL (IQR 2.8-4.2); P<0.001) and higher baseline CAR-HEMATOTOX scores [2 (IQR 1-3.25) vs 1 (IQR 0-1)], consistent with poor baseline hematopoietic reserve (Rejeski, Blood 2021). While disease-related mortality did not significantly differ between groups, non-relapse mortality was markedly higher in patients with long-term B-cell aplasia due to infection: all 8 patient deaths in the aplasia group were due to infections occurring beyond day 30 post-infusion, with 6 of the 8 deaths due to COVID-19. However, no patients in the B-cell recovery group died of late infection (Fig. B).
Conclusions: Long term B-cell aplasia is hypothesized to represent ongoing CAR T-cell activity against normal B-cells. However, our results demonstrate that B-cell aplasia is associated with low baseline hematopoietic reserve and subsequent poor immune reconstitution of multiple lineages including B-cells, T-cells, and myeloid cells. Further studies are warranted to assess whether poor immune reconstitution and B-cell aplasia are related to long term CAR T-cell activity or other processes. These patients are at higher risk for non-relapse mortality from infection, and mitigation strategies are needed.
Disclosures
Perez Perez:Gilead Kite: Speakers Bureau. Bachmeier:Kite Pharma: Consultancy. Shah:Incyte, Jazz Pharmaceuticals, Kite/Gilead, SERVIER: Research Funding; Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite/Gilead: Other: Travel, Accommodations, Expenses; Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences: Honoraria; DSMC, Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Moffitt Cancer Center: Current Employment; Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, Pepromene: Consultancy. Chavez:Epizyme: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Genmab: Honoraria; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Lilly: Honoraria; Merck: Research Funding; Morphosys: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Faramand:Gilead: Research Funding; Kite: Research Funding. Liu:BioLineRx: Membership on an entity's Board of Directors or advisory committees. Lazaryan:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rejeski:Novartis: Honoraria; BMS/CELGENE: Consultancy, Honoraria; Kite/Gilead: Other: Travel Support, Research Funding; Pierre-Fabre: Other: Travel Support. Subklewe:Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead/Kite: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte Biosciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Speakers Bureau; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; AvenCell: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Other: Travel Support, Speakers Bureau; Ichnos Sciences: Consultancy, Honoraria; Seagen: Research Funding; Molecular Partners: Consultancy, Honoraria, Research Funding; GSK: Speakers Bureau; LAWG: Speakers Bureau; Springer Healthcare: Speakers Bureau; AbbVie: Consultancy, Honoraria; Autolus: Consultancy, Honoraria; advesya (CanCell Therapeutics): Consultancy, Honoraria; Genmab US: Consultancy, Honoraria; Interius BioTherapeutics: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Orbital Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Scare: Consultancy, Honoraria. Davila:Precision Biosciences: Other: Ownership interest (stock, stock options in a publicly owned company); Legend Biotech: Consultancy; Kite Pharma Inc.: Other: Teaching and Speaking; Caribou Biosciences: Consultancy; Capstan: Other: Advisor or review panel participant; CRISPR (CRSP): Patents & Royalties: Intellectual property rights (Royalties or patent sales); Bellicum Pharmaceuticals, Inc.: Other: Advisor or review panel participant; Ownership interest (stock, stock options in a publicly owned company); Adicet: Consultancy; Atara Biotherapeutics: Consultancy; Adaptive Biotechnologies: Other: Ownership interest (stock, stock options in a publicly owned company); Syncopation Life Sciences: Consultancy; Synthekine: Consultancy. Locke:Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cowen: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Daiichi Sankyo: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions: Consultancy, Other; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Other; Leukemia and Lymphoma Society: Other; National Cancer Institute: Other; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Institutional; Clinical Care Options Oncology: Other; Pfizer: Membership on an entity's Board of Directors or advisory committees; Caribou: Consultancy; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellular Medicine Group: Consultancy; Imedex: Other; Calibr: Consultancy; CERo Therapeutics: Other: (Institutional); EcoR1: Consultancy; ASH: Other: Travel Support; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Travel Support; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Jain:Kite/Gilead: Consultancy, Honoraria, Research Funding; Loxo@Lilly: Research Funding; Myeloid Therapeutics: Consultancy, Honoraria; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal